
Search
Statistics
We have 222 registered usersThe newest registered user is raheelmemon
Our users have posted a total of 1140 messages in 613 subjects
If you are seeing this, you have attempted to link to the UpToDate widget but are experiencing a problem. Please visit UpToDate for more information.
Phencyclidine Model of Schizophrenia
FORUM FOR PSYCHIATRY RESIDENTS :: Psychiatry :: Psychiatry-Neurology-Psychology discussion :: Psychiatry In Depth
Page 1 of 1
 Phencyclidine Model of Schizophrenia
Phencyclidine Model of Schizophrenia
Phencyclidine Model of Schizophrenia
Schizophrenia is characterized by deficits in cognition known to be dependent upon the functional integrity of the prefrontal cortex (PFC).
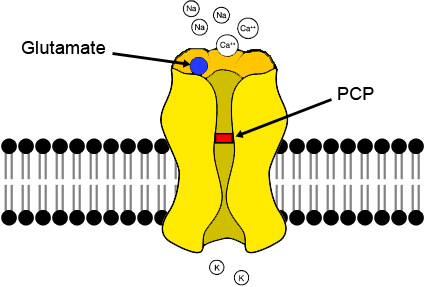
While the pathophysiological basis of PFC dysfunction in schizophrenia is not completely understood, a central role for NMDA receptor hypofunction is widely supported. For example, subchronic exposure to the NMDA receptor antagonist phencyclidine (PCP) induces cognitive deficits and a ‘hypofrontality’ which directly parallels that seen in schizophrenia [1-3].
Furthermore, sub-chronic PCP exposure induces alterations in GABAergic cell markers and 5-HT receptor expression in the PFC similar to those seen in this disorder [1,4,5]. Subchronic exposure to PCP produces a persistent decrease in dopamine utilization within the prefrontal cortex, which is accompanied by a deficit in working memory in prefrontal cortex-dependent tasks in both rats (6) and nonhuman primates (7). These findings have been reviewed by Jentsch and Roth ( 8 ) as supporting NMDA receptor antagonist models of schizophrenia.
While this evidence places NMDA receptor hypofunction central to the pathophysiology of PFC dysfunction in schizophrenia, the mechanisms through which NMDA hypofunction promotes PFC dysfunction are poorly understood.
PCP and its congener, ketamine, have been shown to induce a psychosis in humans that closely resembles schizophrenia and is representative of both the negative and positive symptoms of the disease, and also to exacerbate symptoms in chronic stabilized schizophrenic patients (9-12).
Animal studies have demonstrated that acute treatment with PCP gives rise to an array of symptoms that relate to schizophrenia, including cognitive deficits (6,7,13,14), disruption in sensory motor gating (15) and impaired social interaction (16). Furthermore, it has been demonstrated that some effects of NMDA receptor antagonists (such as neurotoxicity) in rats are age dependent (17,18), which reflects a similar scenario in humans in relation to the onset of sensitivity to the psychotomimetic effects of ketamine (19) and the symptoms of schizophrenia.
Numerous human imaging studies have employed positron emission tomography in schizophrenic patients, either to measure cerebral blood flow or else to measure local cerebral glucose utilization, which is directly related to neuronal activity, with [18F] fluorodeoxyglucose (FDGPET). These studies have frequently revealed an absolute or relative metabolic hypofunction within particular brain areas that have been shown to have altered neuropathology and neurochemical deficits in schizophrenia (Hazlett et al, 2000; Buchsbaum and Hazlett, 1998; Cohen et al, 1997; Buchsbaum et al, 1996; Nordahl et al, 1996; Schroder et al, 1996, 1994; Potkin et al, 1994; Wolkin et al, 1992; Andreasen et al, 1992; Tamminga et al, 1992; Buchsbaum et al, 1990).
In particular, the altered metabolic activity in the prefrontal cortex in the schizophrenic brain has been shown to correlate with the presence and severity of negative symptoms and cognitive deficits (Hazlett et al, 2000; Volz et al, 1999; Buchsbaum and Hazlett, 1998; Schroder et al, 1996; Schroeder et al, 1994; Wolkin et al, 1992; Tamminga et al, 1992; Andreasen et al, 1992; Buchsbaum et al, 1990), while altered metabolic activities within the temporal lobe and thalamus have been shown to correlate with positive symptomology (Buchsbaum and Hazlett, 1998; Buchsbaum et al, 1996; Nordahl et al, 1996; Schroder et al, 1996; Tamminga et al, 1992).
Moreover, chronic exposure to PCP in humans has been shown to produce a metabolic hypofunction during frontal activation tasks (Wu et al, 1991), similar to that observed in schizophrenia, and to produce enduring cognitive deficits (Cosgrove and Newell, 1991). Strikingly, however, chronic PCP treatment has never been shown to produce a neuroanatomical pattern of hypofunction in rodents, which mirrors that observed in schizophrenic patients.
REFERENCES:
1. Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ: Induction of Metabolic Hypofunction and Neurochemical Deficits after Chronic Intermittent Exposure to Phencyclidine: Differential Modulation by Antipsychotic Drugs. Neuropsychopharmacology 2003, 28:265-275.
2. Dawson N, Thompson RJ, McVie A, Thomson DM, Morris BJ, Pratt JA: Modafinil reverses phencyclidine (PCP)-induced deficits in cognitive flexibility, cerebral metabolism and functional brain connectivity. Schizophrenia Bulletin .
3. Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt JA: Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology 2008, 198:37-49.
4. Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA: Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia.
Psychopharmacology 2005, 179:77-84.
5. Steward LJ, Kennedy MD, Morris BJ, Pratt JA: The atypical antipsychotic drug clozapine enhances chronic PCP-induced regulation of prefrontal cortex 5-HT2A receptors. Neuropharmacology 2004, 47:527-537.
6. Jentsch JD, Redmond DE, Elsworth JD, Taylor JR, Youngren KD, Roth RH (1997a). Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277: 953–955.
7. Jentsch JD, Tran A, Le D, Youngren KD, Roth RH (1997b). Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal corticaldependent cognition in the rat. Neuropsychopharmacology 17: 92–99.
8. Jentsch JD, Roth RH (1999). The Neuropsychopharmacology of Phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20: 201–225.
9. Luby ED, Cohen CB, Rosenbaum G, Gottlieb JS, Kelly R (1959). Study of a new schizophrenomimetic drug FSerynl. Arch Neurol Psychiatry 81: 363–369.
10. Allen RM, Young SJ (1978). Phencyclidine-induced psychosis. Am J Psychiatry 135: 1081–1084.
11. Javitt DC, Zukin SR (1991). Recent advances in the phencyclidine model of schizophrenia. AM J Psychiatry 148: 1301–1308.
12. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214.
13. Adams B, Moghaddam B (1998). Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18: 5545–5554.
14. Kesner RP, Dakis M (1997). Intrahippocampal injections of phencyclidine but not naloxone disrupt acquisition of a spatial continuous recognition memory task. Pharmacol Biochem Behav 56: 97–101.
15. Mansbach RS, Geyer MA (1989). Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology 2: 299–308.
16. Sams-Dodd F (1997). Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behav Pharmacol 8: 196–215.
17. Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H et al (1995). Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia?
Biol Psychiatry 38: 788–796.
18. Olney JW, Farber NB (1995). Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52: 998–1007.
19. Reich DL, Silvay G (1989). Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth 36: 186–197.
 Similar topics
Similar topics» DiGeorge Syndrome & Schizophrenia
» PRITE High Yield Topic Discussion Thread
» Schizophrenia Discussion Thread.
» Schizophrenia & Functional Capacity
» Schizophrenia- In Depth Explanation & Discussion
» PRITE High Yield Topic Discussion Thread
» Schizophrenia Discussion Thread.
» Schizophrenia & Functional Capacity
» Schizophrenia- In Depth Explanation & Discussion
FORUM FOR PSYCHIATRY RESIDENTS :: Psychiatry :: Psychiatry-Neurology-Psychology discussion :: Psychiatry In Depth
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum

» L-Methylfolate: Who Will benefit
» Vitamins & Supplements in Clinical Practice.
» Imaging Biomarkers for Outcomes in Mild TBI
» Q.5 Clozapine Neutopenia
» Treating Disorders!
» Cortical Abnormalities in Adults & Adolescents with MDD
» Efficacy of Antipsychotics in Pediatric Acute Mania
» Obsessive Compulsive Disorder in Adults: Which Treatment is Better?