
Search
Statistics
We have 222 registered usersThe newest registered user is raheelmemon
Our users have posted a total of 1140 messages in 613 subjects
If you are seeing this, you have attempted to link to the UpToDate widget but are experiencing a problem. Please visit UpToDate for more information.
Effectiveness of Transcranial Magnetic Stimulation in Clinical Practice Post-FDA Approval
FORUM FOR PSYCHIATRY RESIDENTS :: Psychiatry :: Psychiatry-Neurology-Psychology discussion :: Psychiatry In Depth
Page 1 of 1
 Effectiveness of Transcranial Magnetic Stimulation in Clinical Practice Post-FDA Approval
Effectiveness of Transcranial Magnetic Stimulation in Clinical Practice Post-FDA Approval
Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center.
J Clin Psychiatry 2012;73(4):e567–e573
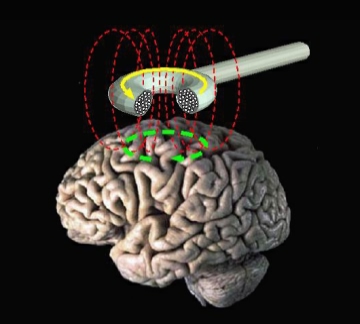
Transcranial magnetic stimulation (TMS) is a US Food and Drug Administration-approved treatment for major depressive disorder (MDD) in patients who have not responded to 1 adequate antidepressant trial in the current episode.
In a retrospective cohort study, we examined the effectiveness and safety of TMS in the first 100 consecutive patients treated for depression (full DSM-IV criteria for major depressive episode in either major depressive disorder or bipolar disorder) at an academic medical center between July 21, 2008, and March 25, 2011.
METHOD:
TMS was flexibly dosed in a course of up to 30 sessions, adjunctive to current medications, for 85 patients treated for acute depression.
The primary outcomes were response and remission rates at treatment end point as measured by the Clinical Global Impressions-Improvement scale (CGI-I) at 6 weeks.
Secondary outcomes included change in the Hamilton Depression Rating Scale (HDRS); Quick Inventory of Depressive Symptomatology, self-report (QIDS-SR); Beck Depression Inventory (BDI); Beck Anxiety Inventory (BAI); and the Sheehan Disability Scale (SDS).
Enduring benefit was assessed over 6 months in patients receiving maintenance TMS treatment.
Data from 12 patients who received TMS as maintenance or continuation treatment after prior electroconvulsive therapy (ECT) or TMS given in a clinical trial setting were also reviewed.
RESULTS:
- The clinical cohort was treatment resistant, with a mean of 3.4 failed adequate trials in the current episode.
- 31 individuals had received prior lifetime ECT, and 60% had a history of psychiatric hospitalization.
- The CGI-I response rate was 50.6% and the remission rate was 24.7% at 6 weeks.
- The mean change was -7.8 points in HDRS score, -5.4 in QIDS-SR, -11.4 in BDI, -5.8 in BAI, and -6.9 in SDS.
- The HDRS response and remission rates were 41.2% and 35.3%, respectively.
- Forty-two patients (49%) entered 6 months of maintenance TMS treatment. Sixty-two percent (26/42 patients) maintained their responder status at the last assessment during the maintenance treatment.
- TMS treatment was well tolerated, with a discontinuation rate of 3% in the acute treatment phase.
- No serious adverse events related to TMS were observed during acute or maintenance treatment.
CONCLUSIONS:
Adjunctive TMS was found to be safe and effective in both acute and maintenance treatment of patients with treatment-resistant depression.
Important points noted in this article:
- Switch from unilateral to bilateral TMS after 10 sessions may be an effective augmentation strategy for slow responders.
- Bipolar depression subgroup had lower response & remission rates compared to unipolar depression subgroup.
- TMS may be helpful for patients who were intolerant (or resistant) to ECT treatment.
 Similar topics
Similar topics» Video: Transcranial Magnetic Stimulation: An Alternative Depression Treatment - Mayo Clinic
» Bipolar Disorder Maintenance Treatment: Monitoring Effectiveness and Safety Michael E. Thase, MD
» Vitamins & Supplements in Clinical Practice.
» CME: Schizophrenia- Translating Science into Clinical Practice
» Clinical Vignette: What is the cause of Nausea & Vomiting?
» Bipolar Disorder Maintenance Treatment: Monitoring Effectiveness and Safety Michael E. Thase, MD
» Vitamins & Supplements in Clinical Practice.
» CME: Schizophrenia- Translating Science into Clinical Practice
» Clinical Vignette: What is the cause of Nausea & Vomiting?
FORUM FOR PSYCHIATRY RESIDENTS :: Psychiatry :: Psychiatry-Neurology-Psychology discussion :: Psychiatry In Depth
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum

» L-Methylfolate: Who Will benefit
» Vitamins & Supplements in Clinical Practice.
» Imaging Biomarkers for Outcomes in Mild TBI
» Q.5 Clozapine Neutopenia
» Treating Disorders!
» Cortical Abnormalities in Adults & Adolescents with MDD
» Efficacy of Antipsychotics in Pediatric Acute Mania
» Obsessive Compulsive Disorder in Adults: Which Treatment is Better?